Damage of amino acids by aliphatic peroxyl radicals: a kinetic and computational study†
Abstract
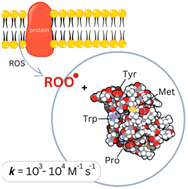
Absolute second-order rate coefficients for the reaction of the N- and C-protected amino acids tyrosine (Tyr), tryptophan (Trp), methionine (Met) and proline (Pro) with triethylamine-derived aliphatic peroxyl radical TEAOO˙, which was used as a model for lipid peroxyl radicals, were determined using laser flash photolysis. For Ac-Tyr-OMe a rate coefficient of 1.4 × 104 M−1 s−1 was obtained, whereas the reactions with Ac-Trp-OMe and Ac-Met-OMe were slower by a factor of 4 and 6, respectively. For the reaction with Ac-Pro-OMe only an upper value of 103 M−1 s−1 could be determined, suggesting that Pro residues are not effective traps for lipid peroxyl radicals. Density functional theory (DFT) calculations revealed that the reactions proceed via radical hydrogen atom transfer (HAT) from the Cα position, indicating that the rate is determined by the exothermicity of the reaction. In the case of Ac-Tyr-OMe, HAT from the phenolic OH group is the kinetically preferred pathway, which shuts down when hydrogen bonding with an amine occurs. In an alkaline environment, where the phenolic OH group is deprotonated, the reaction is predicted to occur preferably at Cβ, likely through a proton-coupled electron transfer (PCET) mechanism.


 Please wait while we load your content...
Please wait while we load your content...