Conjugated structures based on quinazolinones and their application in fluorescent labeling†
Abstract
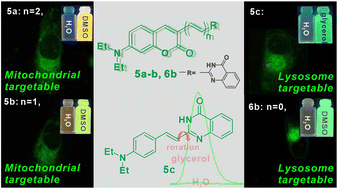
As an alkaloid, quinazolinone exhibits excellent biological properties; structurally, it also has the potential to construct fluorescent probes with conjugated structures. In this work, probes 5a–c and 6b were obtained by introducing quinazolone into aldehydes with different numbers of double bonds. Their absorption maxima were located at 420–540 nm and their emission maxima were at 500–600 nm in solvents of different polarities. In particular, probe 5c showed significant fluorescence enhancement with the increase in viscosity due to the limited intramolecular rotation, and its fluorescence intensity in glycerol was 37.8 times higher than that in water. Moreover, probes 5a–c and 6b containing the NH structure showed sensitive response to pH, and their fluorescence intensity in alkaline solution (pH 9–11) was suddenly enhanced, which was elucidated with the help of theoretical calculation. In addition, the cell experiments showed that probes 5a and 5b had the ability to target mitochondria and probes 5c and 6b targeted lysosomes in HeLa cells. Furthermore, the viscosity-sensitive probe 5c could be used for monitoring changes in lysosomal viscosity in HeLa cells, which had important guiding significance for designing multi-response fluorogenic probes and promoting the advancement of cancer diagnosis.


 Please wait while we load your content...
Please wait while we load your content...