Enzymatic synthesis of amlodipine amides and evaluation of their anti-Trypanosoma cruzi activity†
Abstract
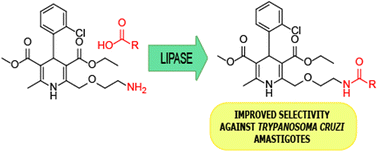
Advancing with our project about the development of new antiparasitic agents, we have enzymatically synthesized a series of amides derived from amlodipine, a calcium channel blocker used as an antihypertensive drug. Through lipase-catalyzed acylation with different carboxylic acids, nineteen amlodipine derivatives were obtained, eighteen of which were new compounds. To optimize the reaction conditions, the influence of several reaction parameters was analyzed, finding different requisites for aliphatic carboxylic acids and phenylacetic acids. All synthesized compounds were evaluated as antiproliferative agents against Trypanosoma cruzi, the etiological agent of American trypanosomiasis (Chagas’ disease). Some of them showed significant activity against the amastigote form of T. cruzi, the clinically relevant form of the parasite. Among synthesized compounds, the derivatives of myristic and linolenic acids showed higher efficacy and lower cytotoxicity. These results added to the advantages shown by the enzymatic methodology, such as mild reaction conditions and low environmental impact, making this approach a valuable way to synthesize these amlodipine derivatives with an application as promising antiparasitic agents.


 Please wait while we load your content...
Please wait while we load your content...