An indole diketopiperazine alkaloid and a bisabolane sesquiterpenoid with unprecedented skeletons from Aspergillus fumigatus†
Abstract
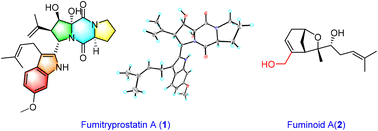
Fumitryprostatin A (1), the first example of an indole diketopiperazine alkaloid with a tricyclic 5/6/5 skeleton characterized by a dipyrrolo[1,2-a:1′,2′-d]pyrazine-5,10-dione ring system decorated with a prenylated indole moiety, and fuminoid A (2), a sesquiterpenoid with a bicyclo[3.2.1]octane ring featuring a novel carbon skeleton via the transformation of the methyl, were isolated from the fungus Aspergillus fumigatus along with six known diketopiperazine alkaloids. The structure with the absolute configuration of 1 was determined based on spectroscopic analyses and X-ray crystallographic analysis, while the configuration of 2 was assigned tentatively by 13C NMR data with DP4+ probability analyses and ECD calculations. A plausible biosynthetic pathway for 1 was proposed starting from L-Trp and L-Pro via normal indole diketopiperazine. Compound 1 exhibited moderate cytotoxic activity with an IC50 value of 14.6 μM, while compound 8 exhibited moderate immunosuppressive activity in vitro.


 Please wait while we load your content...
Please wait while we load your content...