Chiral phosphoric acid-catalyzed enantioselective aza-Friedel–Crafts reaction of naphthols and electron-rich phenols with 2-aryl-3H-indol-3-ones†
Abstract
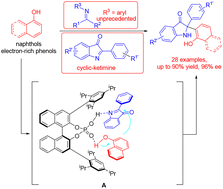
The enantioselective aza-Friedel–Crafts reaction is one of the most straightforward and efficient strategies for constructing a new carbon–carbon bond bearing quaternary stereocenter in organic synthesis, but the catalytic asymmetric aza-Friedel–Crafts reaction of naphthols/phenols with cyclic-ketimines attached to a neutral functional group remains still relatively unexplored. Herein, a highly enantioselective aza-Friedel–Crafts reaction of cyclic-ketimines and naphthols/phenols has been realized using a chiral phosphoric acid catalyst. A variety of chiral aminonaphthols (chiral indolin-3-ones) containing a quaternary stereocenter at the C2 position were obtained with excellent outcomes (up to 97% yield, 98% ee). Moreover, the synthetic utility of the enantiomerically enriched chiral aminonaphthols was demonstrated in some efficient transformations. According to the experimental results, a possible transition state model has been proposed to rationalize the origin of asymmetric induction.


 Please wait while we load your content...
Please wait while we load your content...