Practical synthesis of unsymmetrical disulfides promoted by bromodimethylsulfonium bromide†
Abstract
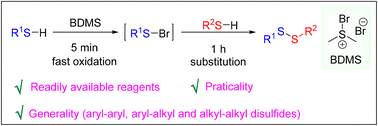
Oxidative cross-coupling of two thiols is the most direct tool for the synthesis of unsymmetrical disulfides and highly desirable across academia and industry. However, the inevitable formation of significant amounts of the corresponding symmetrical by-products is a major issue. We herein present a method toward the synthesis of unsymmetrical disulfides in which the homo-coupling of the thiols is effectively inhibited by adding the two thiols sequentially, taking advantage of rapid oxidation of the thiol by bromodimethylsulfonium bromide.


 Please wait while we load your content...
Please wait while we load your content...