Visible light driven multicomponent synthesis of difluoroamidosulfonyl quinoline derivatives†
Abstract
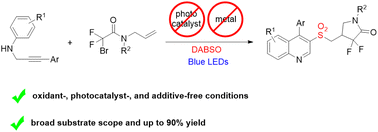
A visible-light-induced photocatalyst-free three-component radical tandem cyclization of N-propargylamine and N-allylbromodifluoroacetamides with the insertion of sulfur dioxide has been developed. Diverse difluoroamidosulfonylated quinolines are obtained in moderate to good yields. This protocol features broad functional group tolerance and high regioselectivity. Moreover, mechanistic studies reveal the involvement of the radical pathway and the formation of an electron donor–acceptor (EDA) complex in this reaction.

 Please wait while we load your content...
Please wait while we load your content...