TsOH-catalyzed acyl migration reaction of the Bz-group: innovative assembly of various building blocks for the synthesis of saccharides†
Abstract
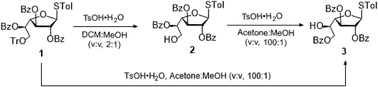
We developed an efficient method to achieve the regioselective acyl migration of benzoyl ester. In all the cases, the reactions required only the commercially available organic acid catalyst TsOH·H2O. This method enables the benzoyl group to migrate from secondary groups to primary hydroxyl groups, or from equatorial secondary hydroxyl groups to axial hydroxyl groups. The 1,2 or 1,3 acyl migration would potentially occur via five- and six-membered cyclic ortho acid intermediates. A wide range of orthogonally protected monosaccharides, which are useful intermediates for the synthesis of natural oligosaccharides, were synthesized. Finally, to demonstrate the utility of the method, a tetrasaccharide portion from a mycobacterial cell wall polysaccharide was assembled.


 Please wait while we load your content...
Please wait while we load your content...