Domino alkyne insertion/aldol reaction/aromatization of 2-alkynyl indole-3-carbaldehyde with 1,3-diketones: entry to 2-indolyl phenols†
Abstract
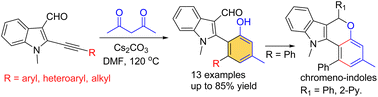
A novel one-pot base-promoted insertion of indolyl 2-alkynes into a C–C single bond of 1,3-diketones, followed by intramolecular aldol reaction and dehydrative aromatization is described. This reaction cascade leads to the construction of 2-indolyl phenols involving the formation of the C1–C2 and C3–C4 bonds of phenols resulting from the formal insertion process with a good substrate scope. Further, these bifunctional compounds were used in a novel arylative annulation in the presence of Grignard reagents to provide chromeno-indole frameworks.


 Please wait while we load your content...
Please wait while we load your content...