Stereoselective synthesis of the spirocyclic core of 13-desmethyl spirolide C using an aza-Claisen rearrangement and an exo-selective Diels–Alder cycloaddition†
Abstract
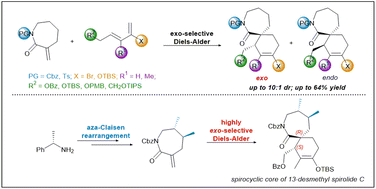
13-Desmethyl spirolide C is a marine natural product of the cyclic imine class that demonstrates remarkable bioactivity against several biomarkers of Alzheimer's Disease, which renders its [7,6]-spirocyclic imine pharmacophore of significant synthetic interest. This work describes a facile and efficient synthesis of the [7,6]-spirocyclic core of 13-desmethyl spirolide C from inexpensive starting materials, featuring an aza-Claisen rearrangement to simultaneously set both stereocentres of the dimethyl moiety with complete atom economy, and a highly exo-selective Diels–Alder cycloaddition to construct the challenging contiguous tertiary and quaternary stereocentres of the spirocyclic core of 13-desmethyl spirolide C. A comprehensive study of the key Diels–Alder reaction was also performed to evaluate the stereoselectivity and reactivity of various functionalised dienes and protected lactam dienophiles, wherein the first successful exo-selective Diels–Alder cycloaddition to construct spirocyclic structures using a bromodiene and α-exo-methylene dienophiles is reported. This strategy not only establishes a more efficient stereoselective access to the spirocyclic core that can be used for the total synthesis of 13-desmethyl spirolide C, but also serves as a sound platform for convenient preparations of a range of spirocyclic analogues required for a comprehensive biological evaluation of this desirable pharmacophore.
- This article is part of the themed collection: Celebrating the 20th anniversary of Organic & Biomolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...