Thermal self-reduction of metal hydroxide acrylate monolayer nanoparticles leads formation of nanoparticulate and porous structured alloys†
Abstract
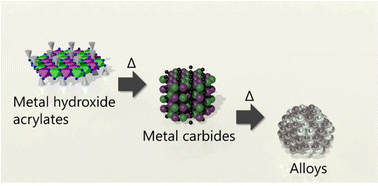
Chemical and physical designs of alloy nanomaterials have attracted considerable attention for the development of highly functional materials. Although polyol processes using ionic precursors are widely used to synthesise alloy nanoparticles, the reduction potential of polyols limits their chemical composition, making it difficult to obtain 3d transition metals. In this study, we employed pre-synthesized metal hydroxide salt monolayer nanoparticles as precursors to obtain alloy nanoparticles. Simultaneous dehydroxylation of the hydroxide moiety and decomposition of the organic moiety allowed the formation of stable face-centred cubic metals passing through the metal carbide and metastable hexagonal close-packed metal phases. This self-reduction process enabled the formation of nanoparticulate bimetallic alloys and macroporous/mesoporous-structured bimetallic alloys by compositing hard/soft templates with pre-synthesized metal hydroxide salt nanoparticles. We believe that the strategy presented in this study can be used to design nanostructures and chemical compositions of multimetallic alloy nanoparticles as well as bimetallic systems.


 Please wait while we load your content...
Please wait while we load your content...