Kinetic versus thermodynamic polymorph stabilization of a tri-carboxylic acid derivative at the solid–liquid interface†
Abstract
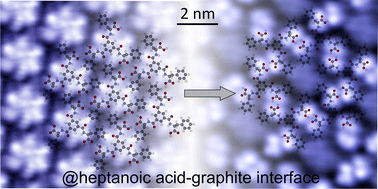
The carboxylic acid moiety gives rise to structural variability in surface-supported self-assembly due to the common expression of various H-bonding motifs. Self-assembly of 3-fold symmetric tricarboxylic acid derivatives on surfaces typically results in monolayer structures that feature the common 2-fold cyclic R22(8) H-bond motif for at least one of the carboxylic acid groups. Polymorphs that are exclusively based on 3-fold cyclic R33(12) H-bonds were predicted but remained elusive. Here, we show the emergence of such a superflower (SF) structure purely based on R33(12) H-bonds for L-benzene-1,3,5-tricarbonyl phenylalanine (L-BTA), a molecule derived from the well-studied trimesic acid (TMA). In contrast to TMA, L-BTA is not completely planar and is also equipped with additional functional groups for the formation of secondary intermolecular bonds. At the heptanoic acid–graphite interface we transiently observe a SF structure, which is dynamically converted into a chicken-wire structure that only exhibits R22(8) H-bonds. Interestingly, when using nonanoic acid as a solvent the initially formed SF structure remained stable. This unexpected behaviour is rationalized by accompanying force field simulations and experimental determination of solvent-dependent L-BTA solubility.


 Please wait while we load your content...
Please wait while we load your content...