Preparation of pure active water for auto-catalytic reactions performed in it†
Abstract
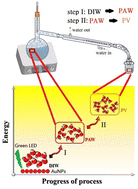
In catalyzed electrochemical reactions, a general strategy is to modify electrode materials to increase the efficiency of the reaction. From the viewpoint of environmental protection, electrochemical reactions should be performed in an inert green water phase. In this study, we report active pure liquid water (named PV), which was collected from the condensed vapor of heated gold (Au)-containing plasmon-activated water (PAW) with a distinct structure of electron-doping and reduced hydrogen bonding (HB). The resulting PV also exhibited distinct properties of the formation of stronger intermolecular HB with alcohols, and notable activities in catalytic electrochemical reactions, compared to bulk deionized water (DIW). Moreover, the measured diffusion coefficients of water increased by ca. 30% in PV solutions. Two typical electrochemical reactions significantly increased peak currents observed in oxidation-reduction cycles (ORCs) with roughening of the Au substrate and in a model of reversible oxidation-reduction reactions on a platinum (Pt) substrate. Also, PV enhanced hydrogen evolution reactions (HERs) on catalytic Pt and inert stainless steel substrates in PV-based solutions at different pH values, compared to DIW. Moreover, these activities of PV were more marked, even better than those of PAW, when PV was collected under a higher heating rate used to heat PAW. Active pure PV has emerged as a promising green solvent applicable to various chemical reactions with more efficiency.


 Please wait while we load your content...
Please wait while we load your content...