Natural sesquiterpene quinone/quinols: chemistry, biological activity, and synthesis†
Abstract
Covering: 2010 to 2021
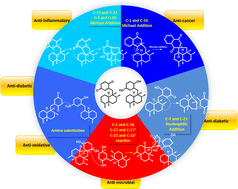
Sesquiterpene quinone/quinols (SQs) are characterized by a C15-sesquiterpenoid unit incorporating a C6-benzoquinone/quinol moiety. Numerous unprecedented carbon skeletons have been constructed with various connection patterns between the two parts. The potent anti-cancer, anti-inflammatory, anti-microbial, anti-viral, and fibrinolytic activities of SQs are associated with their diverse structures. The representative avarol has even entered the stage of clinical phase II research as an anti-HIV agent, and was developed as paramedic medicine against psoriasis. This review provides an overall summary of 558 new natural SQs discovered between 2010 and 2021, including seven groups and sixteen structure-type subgroups, which comprehensively recapitulates their chemical structures, spectral characteristics, source organisms, biological activities, synthesis, and biosynthesis, aiming to expand the application scope of this unique natural product resource.


 Please wait while we load your content...
Please wait while we load your content...