The ‘emodin family’ of fungal natural products–amalgamating a century of research with recent genomics-based advances
Abstract
Covering: up to 2022
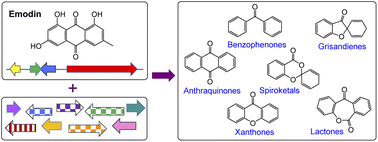
A very large group of biosynthetically linked fungal secondary metabolites are formed via the key intermediate emodin and its corresponding anthrone. The group includes anthraquinones such as chrysophanol and cladofulvin, the grisandienes geodin and trypacidin, the diphenyl ether pestheic acid, benzophenones such as monodictyphenone and various xanthones including the prenylated shamixanthones, the agnestins and dimeric xanthones such as the ergochromes, cryptosporioptides and neosartorin. Such compounds exhibit a wide range of bioactivities and as such have been utilised in traditional medicine for centuries, as well as garnering more recent interest from the pharmaceutical sector. Additional interest comes from industries such as textiles and cosmetics due to their use as natural colourants. A variety of biosynthetic routes and mechanisms have been proposed for this family of compounds, being altered and updated as new biosynthetic methods develop and new results emerge. After nearly 100 years of such research, this review aims to provide a comprehensive overview of what is currently known about the biosynthesis of this important family, amalgamating the early chemical and biosynthetic studies with the more recent genetics-based advances and comparative bioinformatics.
- This article is part of the themed collection: Engineering the biosynthesis of fungal natural products


 Please wait while we load your content...
Please wait while we load your content...