Design, synthesis, molecular docking and molecular dynamic studies of novel benzimidazole–thiazole derivatives as potent and selective COX-2 inhibitors†
Abstract
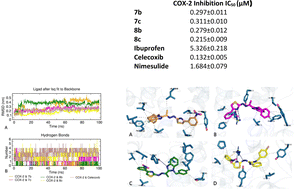
A novel class of benzimidazole–thiazole products have been designed as potential inhibitors of cyclooxygenase, and the synthesized compound structures were verified using instrumental analysis techniques. The in vitro inhibitory effect on COX-1 was dose-dependent, with a significant reduction in effectiveness at lower concentrations. Among the compounds (7b) (IC50 0.297 μM) (7c) (IC50 0.311 μM) (8b) (IC50 0.279 μM) and (8c) (IC50 0.215 μM), which are the most active compounds, IC50 values were close to celecoxib (IC50 0.132 μM) against COX-2. Molecular docking studies were carried out on compounds against COX-2 using Glide XP. The compounds 7b, 7c, 8b, and 8c demonstrated significant docking scores of −8.927 kcal mol−1, −8.578 kcal mol−1, −8.485 kcal mol−1, and −8.899 kcal mol−1, respectively, which suggest a promising interaction with the COX-2 enzyme. Moreover, molecular dynamics simulations of 100 ns duration were performed with COX-2 and active compound complexes using Gromacs. The dynamic simulation results support the findings from the molecular docking studies and suggest that compounds 7b, 7c, 8b, and 8c may have the potential to act as potent and selective COX-2 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...