Regiodivergent sulfonylation of terminal olefins via dearomative rearrangement†
Abstract
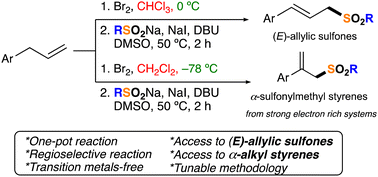
Sulfones are fascinating and highly used functional groups, but current syntheses still have limitations. Here, a regiodivergent transition metal-free approach towards sulfones [(E)-allylic sulfones and α-sulfonylmethyl styrenes] is reported. The method employs commercially available olefins, bases, additives, solvents, and sodium sulfinates (RSO2Na) and produces adducts in good yields. Considering that up to 4 reactions (bromination, dearomative rearrangement, E2, and SN2) are happening, this approach is very efficient. The structures of key adducts were confirmed by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...