Symmetric 4,6-dialkyl/arylamino-5-nitropyrimidines: theoretical explanation of why aminolysis of alkoxy groups is favoured over chlorine aminolysis in nitro-activated pyrimidines†
Abstract
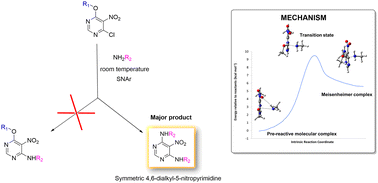
A new synthetic route to obtain symmetric disubstituted alkyl/arylaminopyrimidines under mild conditions is presented, which can be used to generate new purine libraries for drug discovery. We investigated the unexpected reaction of 6-alkoxy-4-chloro-5-nitropyrimidines with primary amines, which produced disubstituted dialkyl/arylamine pyrimidines instead of the expected 6-alkoxy-4-alkylamine-5-nitropyrimidines. To clarify this reaction, a computational study of the reaction mechanism was carried out. Our results suggest that the presence of pre-reactive molecular complexes, a phenomenon rarely reported in SNAr reactions, precedes the transition state and can facilitate the reaction. In addition, Meisenheimer complexes and transition states in the intrinsic reaction coordinate (IRC) configuration, which may influence the understanding of the reaction mechanism, were identified.


 Please wait while we load your content...
Please wait while we load your content...