Mechanistic insights into chloramphenicol-mediated inactivation of cytochrome P450 enzymes and their active site mutants†
Abstract
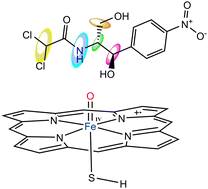
Drugs that inhibit cytochrome P450 (P450) enzymes through mechanism-based inactivation (MBI) can significantly affect metabolic interactions, often leading to adverse effects. Despite this, the MBI of P450 is not an uncommon occurrence, as evidenced by numerous previous studies. In our investigation, we used Density Functional Theory (DFT) calculations to delve into the MBI of P450 by chloramphenicol (CP), evaluating the energy profiles of five possible pathways for this process. Furthermore, we conducted a multitargeted molecular modeling analysis to discern the binding profile of CP at the active site of five distinct P450 isozymes. Our molecular modeling results reveal that CP demonstrates a high degree of compatibility with various P450 isoforms’ binding sites, owing to its low binding energies, the presence of a dense network of polar and hydrophobic interacting residues, and the involvement of key residues in the binding interactions. Our DFT calculations suggest that CP is a potent P450 inhibitor, primarily due to the availability of two thermodynamically favored interaction sites on CP structure vulnerable to P450 interaction. Our comparative analysis indicates that P450's inactivation by CP principally proceeds via the C–H hydroxylation of the dichloromethane and hydroxymethylene binding sites present in the CP structure.


 Please wait while we load your content...
Please wait while we load your content...