Synthesis of fluorescent aromatic species via ring transformation of α-pyranones and their application in OLEDs, chemosensors, and cell imaging
Abstract
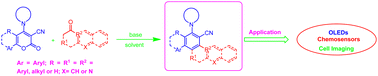
Donor–acceptor substituted aromatic systems are highly regarded organic fluorophores with unique photophysical and electrochemical properties. These molecules offer a wide range of opportunities for advancing modern science through easily modifiable molecular structures. In recent years, there has been growing interest in developing convenient methodologies for synthesising these fluorescent compounds. The carbanion-induced ring transformation of α-pyranones is one such approach that has yielded numerous D–π–A dyads based on aromatic systems with widespread applications as effective fluorophores. Moreover, this method provides explicit functionalization options, allowing fine-tuning of optoelectronic properties according to specific requirements without requiring complex reaction setups or transition metal catalysts/reagents, which makes it more advantageous over other conventional approaches. This review discusses the synthesis of polycyclic aromatic compounds functionalized with donor–acceptor groups via ring transformation reactions of α-pyranone derivatives induced with various carbon nucleophiles, along with their applications in OLEDs, chemosensors, and cell imaging.
- This article is part of the themed collection: 2023 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...