Michael addition-driven synthesis of cytotoxic palladium(ii) complexes from chromone thiosemicarbazones: investigation of anticancer activity through in vitro and in vivo studies†
Abstract
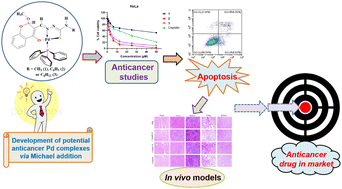
Three Pd(II) complexes (1–3) containing tridentate chromone-based thiosemicarbazones (SVSHL1–3) have been investigated as potential anticancer agents. The complexes of the type [Pd(PPh3)(ONS-(SVSHL1–3)-OCH3)] were synthesized by the reaction of [PdCl2(PPh3)2] with the corresponding TSC ligands under inert conditions; the formation of the complexes occurred with the ligands undergoing in situ Michael addition. The characterization of complexes was achieved with the aid of analytical and various spectroscopic techniques. The molecular structure of complex 3 was determined using a single crystal X-ray diffraction (XRD) method, confirming the Michael addition pathway as well. The complexes were screened against a panel of cancer and normal cell lines to evaluate their anticancer activity; complexes 2 and 3 with a phenyl or cyclohexyl substituent on the N-terminal of the ligands exhibited IC50 values of 2.87 and 4.01 μM against HeLa cancer cells, respectively. The apoptotic mode of cell death was confirmed using microscopic and flow cytometry studies. Interestingly, normal cell architecture was seen in the tissues of mice treated with the active Pd(II) complexes, in contrast with those treated with cisplatin which induced severe damage to the normal tissues in mice.


 Please wait while we load your content...
Please wait while we load your content...