Fabrication of Schottky barrier diodes utilizing carboxylate bridged trinuclear mixed valence cobalt(iii/ii/iii) complexes of tetradentate N2O2 donor reduced Schiff base ligands†
Abstract
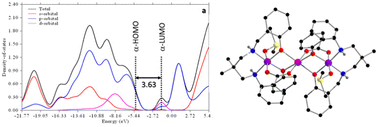
Three mixed-valence trinuclear cobalt(III)–cobalt(II)–cobalt(III) complexes, [CoII{(μ-L1)(μ-OAc)CoIII(OAc)}2]·2.67H2O (1), [CoII{(μ-L1)(μ-OOCPh)CoIII(DMSO)0.8(OOCPh)0.2}2](ClO4)1.6 (2) and [CoII{(μ-L2)(μ-OOCPh)CoIII(DMSO)0.75(OOCPh)0.25}2](ClO4)1.5 (3), have been synthesized using two tetradentate N2O2 donor ‘reduced Schiff base’ ligands, H2L1 {1,3-bis(2-hydroxybenzylamino)2,2-dimethylpropane} and H2L2 {2,2′-[1,1′-(propane-2,2-diyldiimino)diethylidene]diphenol}, and acetate or benzoate as anionic co-ligands. These complexes have been characterized by spectroscopic measurements and their solid state structures have been determined by single crystal X-ray diffraction analysis. The cobalt(III)–cobalt(II)–cobalt(III) skeletons of complexes 1 and 2 are linear, centrosymmetric; but in complex 3, it is non-centrosymmetric. Both the central cobalt(II) and terminal cobalt(III) atoms are hexa-coordinated in all three complexes. The structure directing role of several noncovalent interactions in the solid state of the complexes has been investigated focusing on the H-bonding interactions mediated by the co-crystallized water molecules in 1 and the perchlorate counterions in 2 and 3. Thermo-gravimetric analysis (TGA) provided a thermal driven pathway to observe the loss of lattice water molecules from complex 1. All the complexes show good electrical conductivity, which has been rationalized by band gap measurements. The band gap in the solid state was determined experimentally and also by DFT calculations and it confirms that each of the complexes behaves as a semiconductor. These complexes have been used to fabricate Schottky barrier diodes.


 Please wait while we load your content...
Please wait while we load your content...