Site-selective nucleophilic substitution reactions of pentafluoropyridine with hydroxybenzaldehydes: synthesis of triarylmethanes comprising perfluoropyridine moieties†
Abstract
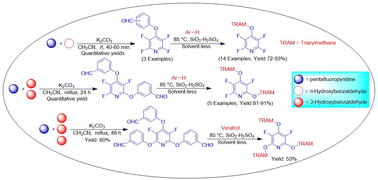
First, the nucleophilic substitution reactions of pentafluoropyridine (PFP) with hydroxybenzaldehydes were evaluated. It was demonstrated that parameters such as reaction conditions, molar ratio of reactants and nucleophilicity of the derived potassium formylphenolates have decisive roles in determining the selectivity of reactions. The nucleophilic attack of hydroxybenzaldehydes under mildly basic conditions took place solely at the C–4 position of PFP and the corresponding 4-((perfluoropyridin-yl)oxy)benzaldehydes 3a–c were obtained in nearly quantitative yields. However, 3-hydroxybenzaldehyde was found to be an effective nucleophile for the selective replacement of fluorine atoms at C–2 and/or C–6 positions of PFP under harsh conditions. While the controlled site-selective reactions of PFP with two-fold amounts of 3-hydroxybenzaldhyde under reflux conditions afforded 3,3′-((3,5,6-trifluoropyridine-2,4-diyl)bis(oxy))dibenzaldehyde 4a in excellent yield, their reaction in a 1 : 3 molar ratio led to producing 3,3′,3′′-((3,5-difluoropyridine-2,4,6-triyl)tris(oxy))tribenzaldehyde 4b in moderate yield. 3,3′-((3,5-Difluoro-4-(4-formylphenoxy)pyridine-2,6-diyl)bis(oxy))dibenzaldehyde, as a mixed-analogue of 4b, was also prepared employing a two-step strategy. Finally, a novel class of mono-, bis-, and tris(triarylmethane) analogues containing perfluoropyridine subunits 9–10 were synthesized via the SiO2–H2SO4 catalysed Friedel–Crafts alkylation reaction of various arenes and heteroarenes with the derived (oxy)benzaldehydes, yet the selective synthesis of unsymmetrical TRAMs 11a and b applying this procedure was unsuccessful.


 Please wait while we load your content...
Please wait while we load your content...