Additive-free reductive hydrodeoxygenation of fatty acids catalyzed by inexpensive simple nickel(ii) compounds†
Abstract
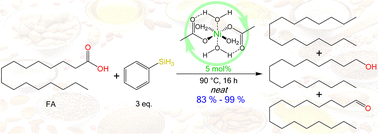
The hydrodeoxygenation (HDO) of different fatty acids (FAs) was achieved using simple and commercially available nickel(II) compounds, with silanes as reducing agents under relatively mild reaction conditions. Thus, the optimized conditions include Ni(AcO)2·4H2O (5 mol%) as a catalytic precursor and 3 eq. of PhSiH3 at 90 °C, 16 h, and the corresponding FAs, such as palmitic acid (PA), stearic acid (SA) and oleic acid (OA), having high conversion (83% to ≥99%) and good selectivity, being specific for the reduction of the carboxylic group to yield the corresponding aldehydes, alcohols, and hydrocarbons through hydrosilylation with PhSiH3 by fine-tuning of the reaction conditions and the use of relatively mild conditions, without requiring additives. To gain insight into the reaction mechanism, the reaction intermediate [Ni(C15H31COO)2(H2O)2] was synthesized, characterized, and tested as an active catalyst.


 Please wait while we load your content...
Please wait while we load your content...