Graphene oxide promotes aggregation-induced emission in binary solvent mixtures†
Abstract
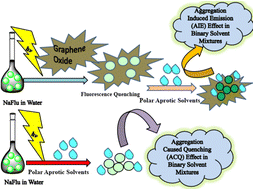
The role of graphene oxide (GO) in quenching the emission properties of various organic fluorophores is well known. However, promoting prototropism of various organic fluorophores or inducing fluorescence enhancement is less documented in the literature. The role of GO on the emission properties of fluorophores in binary-solvent mixtures is not very well studied. In this article, we report the photophysical behavior of fluorescein (NaFlu) molecules in the presence as well as in the absence of GO, in neat solvents and water–polar aprotic solvent binary mixtures. In the presence of GO, addition of polar aprotic solvents promotes solvent-induced aggregation of NaFlu in water, i.e. aggregation-induced emission (AIE), since due to the aggregate formation there is a restriction in the intramolecular rotation of NaFlu molecules, and the chances of non-radiative relaxation pathways decreases. In the absence of GO, NaFlu forms aggregates which leads to π–π coplanar interactions between NaFlu molecules leading to excimer species formation that prefers to decay through non-radiative pathways thus promoting an aggregation-caused quenching (ACQ) phenomenon. Therefore, the presence of GO facilitates the AIE of NaFlu molecules in different binary solvent mixtures. This property of GO will be helpful to create novel GO nanocomposites for device and biosensor applications.
- This article is part of the themed collection: New Journal of Chemistry Selected Articles in Physical and Materials Chemistry from India


 Please wait while we load your content...
Please wait while we load your content...