Degradation processes of protic ionic liquids for NH3 separation†
Abstract
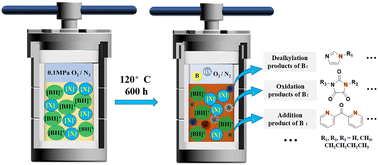
Protic ionic liquids (PILs) have been the focus of research both domestically and internationally because PILs have demonstrated improved NH3 separation capabilities and have been applied to separate NH3 from industrial tail gas. As the stabilities of PILs are overestimated, it is important to study their long-term stability as well as the degradation mechanism of PILs to slow down their degradation and improve their long-term stability. In this work, degradation experiments on 7 PILs were carried out at up to 120 °C for 600 h in N2 and O2 atmospheres. The impacts of different anions, cations, and NH3 contents on the degradation processes of PILs were explored. High-performance liquid chromatography (HPLC) was used to quantify the degradation ratios of the PILs. The degradation products of PILs were identified and quantified via gas chromatography (GC)/mass spectrometry (MS), and the degradation mechanisms of the PILs were extrapolated according to the degradation products. Our results revealed that degradation occurred mainly in the cations, although the anions also affected the degradation processes of the PILs. The degradation ratios of the PILs decreased with an increase in the NH3 content. This study on the degradation processes of PILs provides theoretical support for the industrial applications of PILs in NH3 separation.


 Please wait while we load your content...
Please wait while we load your content...