Synthesis of 1,4-benzoxazine derivatives from α-aminocarbonyls under transition-metal-free conditions†
Abstract
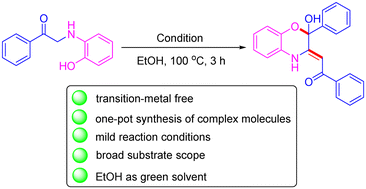
Herein, we report a one-pot tandem reaction for the synthesis of 1,4-benzoxazine derivatives with up to 83% yield. The present protocol, using ethanol as a solvent and avoiding the use of transition metal catalysts, exhibits a wide range of substrate scope and functional group tolerance. This novel, mild and efficient method is highly promising for constructing 1,4-benzoxazine derivatives.


 Please wait while we load your content...
Please wait while we load your content...