A covalent organic polymer containing nitrogen and oxygen groups with high adsorption capacity and selectivity for gold ions under strongly acidic conditions†
Abstract
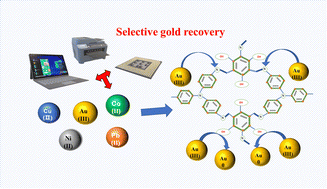
With the widespread use of gold as a precious metal, the recovery of gold has become an urgent problem to be solved. Therefore, the design and preparation of a sorbent with better adsorbability and higher selectivity for Au(III) is of great significance for recovering gold resources and protecting the environment. In order to obtain an efficient and stable gold ion sorbent, N1,N1′-(1,4-phenylene)bis(N1-(4-aminophenyl)benzene-1,4-diamine) and 2,4,6-tricarboxylisophene were cross-linked by a solvent thermal reaction to synthesize a double-functional covalent organic polymer (N-TPCOP). The N-TPCOP has a very high gold uptake capacity of 3400.38 mg g−1, because it reduces Au(III) to elemental gold by sequestration and redox reactions between functional atoms (N and O) and Au(III). In summary, we have verified the adsorption potential of a novel covalent organic polymer for Au(III), which provides a strong reference for the recycling of precious metal gold in secondary resources and the protection of environmental resources.


 Please wait while we load your content...
Please wait while we load your content...