Cyclometallated iridium NHC complexes containing self-isomerised ligands as catalysts for hydrosilylation and transfer hydrogenation reactions†‡
Abstract
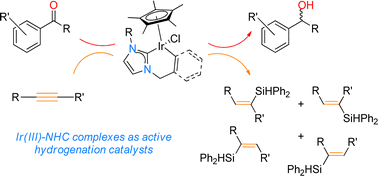
Novel Ir-based NHC complexes (1–4) were synthesised using N-alkenyl functionalised imidazolium salts with Ir(III) precursors. Complex 1 represents an unprecedented cyclometallated complex, which is the first and only example in literature, to our knowledge, of a non-aromatic C(sp2)–H activation leading to a C(sp3)–Ir cyclometallated product. A concomitant intramolecular/isomerisation process was followed by η3-allyl bond formation to a second Ir centre, thus forming a homonuclear bimetallic complex. Subsequent reactions with a range of imidazolium salts featuring different N-pendant arms were performed, including alkenyl and benzyl tethers. Selectivity with regards to cyclometallation behaviour of the iridium centre was observed in preference for a benzyl cyclometallated product over alkenyl cyclometallation. Benzyl-containing products proved most promising as active catalysts in both the hydrosilylation of internal alkynes with a conversion of up to 59% after 1 hour, as well as the transfer hydrogenation of ketone and aldehyde substrates with conversions of up to 100% after 18 hours using catalyst loadings of 4 and 1 mol% respectively.


 Please wait while we load your content...
Please wait while we load your content...