Rapid alcoholysis of cyclic esters using metal alkoxides: access to linear lactyllactate-grafted polyglycidol†
Abstract
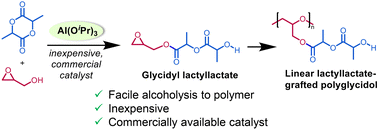
A facile alcoholysis of L-lactide (LA) and lactones was developed using inexpensive and commercially available Al(OiPr)3 (1), Ti(OiPr)4 (2), and Zr(OiPr)4.iPrOH (3) yielding alkyl (S,S)-lactyllactates and hydroxy esters. Complex 1 was the most efficient for the methanolysis of LA at 70 °C finishing very rapidly within 10 min selectively giving 99% methyl (S,S)-lactyllactate. The alcoholysis can be extended efficiently to other functional alcohols containing triple bonds, and methacrylate, and epoxide groups. The alcoholysis of LA by 1 with glycidol gave glycidyl (S,S)-lactyllactate as a new epoxide monomer in excellent yield. This monomer can be polymerized selectively at the epoxide group by 1 giving a novel linear polyglycidol having precisely one biodegradable lactyllactate unit grafted on each glycidol molecule that has not been accessible by previous methods.


 Please wait while we load your content...
Please wait while we load your content...