Design, synthesis and biological evaluation of novel potent STAT3 inhibitors based on related heterocycle-fused naphthoquinones for cancer therapy†
Abstract
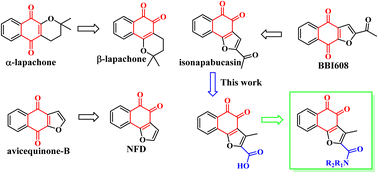
Persistently activated signal transducer and activator of transcription 3 (STAT3) plays an important role in the development of multiple cancers, and therefore it is a potential therapeutic target for cancer treatment. Herein, we report the rational design, synthesis, and biological evaluation of novel potent STAT3 inhibitors based on iso-napabucasin. Among them, compound A14 exhibited the most potent in vitro tumor cell growth inhibitory activities against HepG2 and K562 cells with IC50 values as low as 0.88 μM and 2.16 μM, respectively. Compound A14 has a moderate percentage of plasma protein binding in vitro (88.6%), good plasma stability and solubility. After oral administration, the bioavailability of A14 was 44.22%. Finally, molecular docking and WB studies further clarified the binding mode of A14 in STAT3 SH2 domain.


 Please wait while we load your content...
Please wait while we load your content...