Preparation of ZrO2via a microwave-assisted hydrothermal method and its catalytic performance for n-pentanal self-condensation†
Abstract
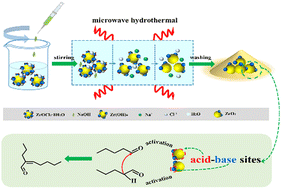
ZrO2 was prepared by a microwave-assisted hydrothermal method, and used as a green and efficient catalyst for self-condensation of n-pentanal to 2-propyl-2-heptenal. The effects of preparation conditions on its structure and acid–base properties were investigated with the assistance of XRD, TEM, BET, Raman spectroscopy, FTIR spectroscopy and TPD techniques. The results demonstrate that tetragonal phase ZrO2 (t-ZrO2) and monoclinic phase ZrO2 (m-ZrO2) coexist in the samples. The enhancement of microwave temperature, power and time can all facilitate the formation of t-ZrO2, whereas the content of t-ZrO2 first increases and then decreases with increasing pH value. Both the content of t-ZrO2 and acid–base properties of ZrO2 can correlate well with its catalytic performance. Additionally the equal acid–base centers and the synergetic catalysis of ZrO2 also account for its high catalytic performance. The n-pentanal conversion and 2-propyl-2-heptenal selectivity reached 88.4% and 97.1%, respectively. Finally, an acid–base synergetic mechanism of ZrO2 for n-pentanal self-condensation was proposed.


 Please wait while we load your content...
Please wait while we load your content...