Unraveling the reaction mechanism of AlCl3 Lewis acid catalyzed acylation reaction of pyrene from the perspective of the molecular electron density theory†
Abstract
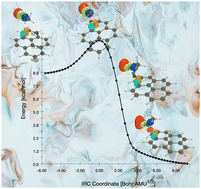
The reaction mechanism of AlCl3 Lewis acid catalyzed acylation of pyrene with the methylacylium ion has been carried out at the ωB97X-D/6-311G(d,p) level within the Molecular Electron Density Theory (MEDT). Before completing this work, CDFT (Conceptual DFT) analysis allows the classification of both reactants as a nucleophile (pyrene) and super electrophile (methylacylium ion), respectively, permitting this acylation to take place with a polar character. This polar character has been supported by the high value of Global Electron Density Transfer (GEDT) recorded for each transition state. Concerning the bond changes, Bonding Evolution Theory (BET) analysis reveals that a series of four Structural Stability Domains (SSDs) are required to describe the formation of new C–C and H–Cl single bonds. For the formation of C–C single bond due to the attack of the methylacylium ion on pyrene, the first two stages correspond to (1) the creation of a pseudoradical center on both C carbon atoms (appearance of a V(C) basin) of each reactant and (2) the creation of a new C–C single bond via the merger of these two previous V(C) basins. Finally, the formation of a H–Cl molecule occurs via cleavage of an H–C bond (splitting of a V(H,C) basin) and the formation of a new H–Cl single bond (appearance of a new V(H,Cl) disynaptic basin), and the last step illustrates the restoration of the aromaticity of the ring engaged in the acylation reaction.


 Please wait while we load your content...
Please wait while we load your content...