Novel (E)-3-(1-substituted-1H-indazol-5-yl)-N-hydroxypropenamides as histone deacetylase inhibitors: design, synthesis and structure–activity relationships†
Abstract
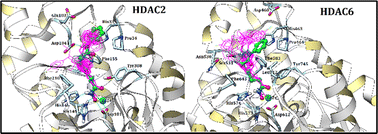
Histone deacetylases are one of the most interesting targets for anticancer drug discovery and developments. Herein, we report the design, synthesis and evaluation of novel N-hydroxypropenamides bearing indazole moieties as potent HDAC inhibitors and anticancer agents. As a result, 18 new compounds were designed and synthesized. The biological evaluation revealed that compounds 5a–f and 7a–f (IC50 values ranging from 0.126 to 3.750 μM) showed comparable activity to the reference compound SAHA (IC50 values ranging from 0.128 to 0.716 μM) in terms of cytotoxicity, as well as HDAC inhibition. In addition, the synthesized compounds showed 2- to 30-fold more potent inhibitory activity against HDAC6 in comparison to that of a mixture of HDAC isoforms in the HeLa cell nuclear extract. Docking studies were conducted to decipher the structure–activity relationships. The results were useful for class IIb isoform-selective inhibitor optimization. Finally, physicochemical and ADMET predictions for 5b and 7e were they have high absorption ability, low metabolic activity and low toxicity, making them promising candidates for further drug discovery analysis.


 Please wait while we load your content...
Please wait while we load your content...