In situ growth of Ni/Fe hydroxide nanosheets using a self-sacrificial template as an efficient and robust electrocatalyst for the oxygen evolution reaction†
Abstract
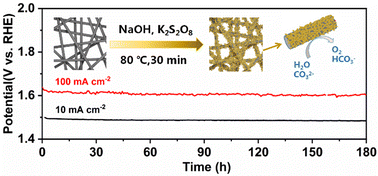
To solve the problem of excessive energy consumption in the process of catalytic water splitting, it is quite essential to exploit high-activity and low-cost electro-catalysts. The present paper proposes a highly catalytically active Ni/Fe hydroxide electro-catalyst for the oxygen evolution reaction (OER), which is in situ formed on the surface of the stainless steel fiber felt using a sacrificial template by adding the oxidizing agent K2S2O8 to the alkaline solution under mild conditions. The electrode prepared using this simple one-step chemical oxidation method obviously shows enhanced catalytic activity for the OER. The overpotentials at 10 mA cm−2 are only 214 mV and 263 mV in KOH and Na2CO3/NaHCO3 solutions, respectively. In addition, the potential and morphology of the electrode remain stable for 180 h at 10 and 100 mA cm−2 in the Na2CO3/NaHCO3 system, demonstrating its ultra-high stability. This new route to prepare OER electrode materials has a promising bright application prospect for large-scale production.


 Please wait while we load your content...
Please wait while we load your content...