Unconventional sulfur transfer behaviour of 4-hydroxy-dithiocoumarin: an easy access to biologically potent 1,2-dithiolane scaffolds†
Abstract
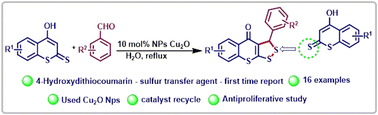
The synthesis of hitherto unreported 3-aryl-3H,4H-[1,2]dithiolo[3,4-b]thiochromen-4-ones was accomplished via the reaction of 4-hydroxydithiocoumarin and an aromatic aldehyde using 10 mol% Cu2O nanoparticles in water under reflux conditions. Due to the unusual sulfur transfer behaviour of 4-hydroxydithiocoumarin, the new class of compounds are easily accessible. The novelty of the present method is due to environmentally benign reaction conditions, fairly good yield, aqueous conditions, and recyclable catalysts. Additionally, the synthesized 1,2-dithiolane compounds (3d, 3f, 3g, 3h & 3i) were investigated for anti-proliferative activities.


 Please wait while we load your content...
Please wait while we load your content...