Palladium-catalyzed decarboxylative cyclization of α-acyloxyketones having an allene moiety in the tether†
Abstract
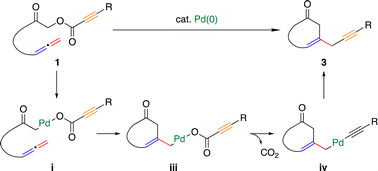
We herein report palladium(0)-catalyzed decarboxylative cyclization of α-acyloxyketones having an allene moiety in the tether, giving various cyclohexanone derivatives. DFT calculations suggested that the reaction proceeds in the following sequence: (1) oxidative addition of the C(α)–O bond of α-acyloxyketones 1 to a palladium(0) complex; (2) insertion of the terminal allene double bond into the Pd–C(sp3) bond to form a C–C bond between the α-carbon of the carbonyl group and the β-carbon in the allene; (3) decarboxylation; and (4) reductive elimination to form a C(sp3)–C(sp) bond. The DFT calculations also rationalized why the reaction gives a 6-membered product instead of a 5- or 7-membered product.


 Please wait while we load your content...
Please wait while we load your content...