Theoretical exploration to the significance of n(S)/n(O) → σ* (C-COOMe) stereoelectronic interactions†
Abstract
α-Heterosubstituted 1,3-dioxane and 1,3-dithiane systems have been extensively reported for 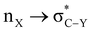 (X, Y = heteroatoms) hyperconjugative interactions. However, the definitive interpretation for the conformational analysis of 2-carbomethoxy 1,3-diheterocyclohexane is less explored. The weaker accepting ability of C–C(carbomethoxy) antibonding orbitals in 2-substituted 1,3-diheterocyclohexane makes it an attractive candidate for estimating the role of stereoelectronic effects in such systems. This study explores the conformational preferences of 2-carbomethoxy substituted 1,3-dioxane, 1,3-oxathiane and 1,3-dithiane in the light of stereoelectronic interactions and the contribution of other factors to attain stability in the respective systems using the CBS-QB3 method, at the MP2/6-311+G(d,p)/SMD(chloroform) and B3LYP/6-311+G(d,p)/SMD(chloroform) levels. The DFT calculated results in the solvent phase reveal that the delocalizing interactions operate in all three systems examined; however, ring strain overrides the axial preference of the 2-carbomethoxy substituent on 1,3-dioxane (2-ax) and 1,3-oxathiane (3-ax). The hyperconjugative interaction, lower ring strain and attractive C–H⋯O non-bonded interactions favor the axial (4-ax) over the equatorial (4-eq) isomer of methyl-2-carboxylate-1,3-dithiane.
(X, Y = heteroatoms) hyperconjugative interactions. However, the definitive interpretation for the conformational analysis of 2-carbomethoxy 1,3-diheterocyclohexane is less explored. The weaker accepting ability of C–C(carbomethoxy) antibonding orbitals in 2-substituted 1,3-diheterocyclohexane makes it an attractive candidate for estimating the role of stereoelectronic effects in such systems. This study explores the conformational preferences of 2-carbomethoxy substituted 1,3-dioxane, 1,3-oxathiane and 1,3-dithiane in the light of stereoelectronic interactions and the contribution of other factors to attain stability in the respective systems using the CBS-QB3 method, at the MP2/6-311+G(d,p)/SMD(chloroform) and B3LYP/6-311+G(d,p)/SMD(chloroform) levels. The DFT calculated results in the solvent phase reveal that the delocalizing interactions operate in all three systems examined; however, ring strain overrides the axial preference of the 2-carbomethoxy substituent on 1,3-dioxane (2-ax) and 1,3-oxathiane (3-ax). The hyperconjugative interaction, lower ring strain and attractive C–H⋯O non-bonded interactions favor the axial (4-ax) over the equatorial (4-eq) isomer of methyl-2-carboxylate-1,3-dithiane.



 Please wait while we load your content...
Please wait while we load your content...