Seed free synthesis of polyethylene glycol stabilized gold nanoprisms exploiting manganese metal at low pH†
Abstract
Manganese powder with a suitable potential (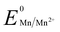 , −1.19 V) has never been investigated for the reduction of Au3+ (
, −1.19 V) has never been investigated for the reduction of Au3+ (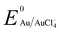 , 1.00 V). In this study, we have utilized
, 1.00 V). In this study, we have utilized 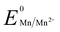 and low pH dependent
and low pH dependent 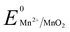 for the polyethylene glycol stabilized gold nanoprism synthesis by reducing AuCl−4 in the presence of thiol terminated polyethylene glycol as the stabilizing agent. The synthetic methodology for gold nanoprisms has been optimized by pH and Cl− ion combination. Time dependent absorbance studies have been conducted to demonstrate the role of various reaction parameters such as the stabilizing agent, HCl concentration, temperature, and Mn metal. The synthesized gold nanoprism has been further utilized as a seed for nucleic acid and selected amino acid mediated edge and surface growth, respectively.
for the polyethylene glycol stabilized gold nanoprism synthesis by reducing AuCl−4 in the presence of thiol terminated polyethylene glycol as the stabilizing agent. The synthetic methodology for gold nanoprisms has been optimized by pH and Cl− ion combination. Time dependent absorbance studies have been conducted to demonstrate the role of various reaction parameters such as the stabilizing agent, HCl concentration, temperature, and Mn metal. The synthesized gold nanoprism has been further utilized as a seed for nucleic acid and selected amino acid mediated edge and surface growth, respectively.



 Please wait while we load your content...
Please wait while we load your content...