Physicochemical properties of different crystal forms of manganese dioxide prepared by a liquid phase method and their quantitative evaluation in capacitor and battery materials
Abstract
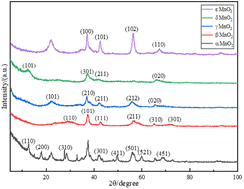
Although there are many studies on the preparation and electrochemical properties of the different crystal forms of manganese dioxide, there are few studies on their preparation by a liquid phase method and the influence of their physical and chemical properties on their electrochemical performance. In this paper, five crystal forms of manganese dioxide were prepared by using manganese sulfate as a manganese source and the difference of their physical and chemical properties was studied by phase morphology, specific surface area, pore size, pore volume, particle size and surface structure. The different crystal forms of manganese dioxide were prepared as electrode materials, and their specific capacitance composition was obtained by performing CV and EIS in a three-electrode system, introducing kinetic calculation and analyzing the principle of electrolyte ions in the electrode reaction process. The results show that δ-MnO2 has the largest specific capacitance due to its layered crystal structure, large specific surface area, abundant structural oxygen vacancies and interlayer bound water, and its capacity is mainly controlled by capacitance. Although the tunnel of the γ-MnO2 crystal structure is small, its large specific surface area, large pore volume and small particle size make it have a specific capacitance that is only inferior to δ-MnO2, and the diffusion contribution in the capacity accounts for nearly half, indicating it also has the characteristics of battery materials. α-MnO2 has a larger crystal tunnel structure, but its capacity is lower due to the smaller specific surface area and less structural oxygen vacancies. ε-MnO2 has a lower specific capacitance is not only the same disadvantage as α-MnO2, but also the disorder of its crystal structure. The tunnel size of β-MnO2 is not conducive to the interpenetration of electrolyte ions, but its high oxygen vacancy concentration makes its contribution of capacitance control obvious. EIS data shows that δ-MnO2 has the smallest charge transfer impedance and bulk diffusion impedance, while the two impedances of γ-MnO2 were the largest, which shows that its capacity performance has great potential for improvement. Combined with the calculation of electrode reaction kinetics and the performance test of five crystal capacitors and batteries, it is shown that δ-MnO2 is more suitable for capacitors and γ-MnO2 is more suitable for batteries.


 Please wait while we load your content...
Please wait while we load your content...