An experimental evidence for the key role of diphenylalanine in fibril formation†
Abstract
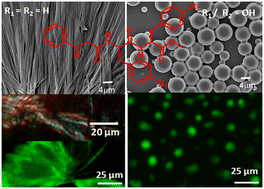
This study examined the role of diphenylalanine (the central hydrophobic cluster of Alzheimer's β-amyloid peptide) in the intermolecular hydrogen-bonded supramolecular sheet and fibril formation. N-phenylglycine appended peptide NPG-Phe-Phe-OMe (1), having a sequence similarity with a diphenylalanine motif, self-aggregates to form entangled fibers. The fibers show green-gold birefringence in a Congo red assay. Moreover, the fibers bind with thioflavin T (ThT) and show an enhanced emission. However, the tyrosine-modified analogues failed to form fibers and rather exhibited a microsphere-like morphology. From X-ray diffraction analysis, the tyrosine-modified analogues, namely, NPG-Phe-Tyr-OMe (2) and NPG-Tyr-Phe-OMe (3), adopt extended conformations and self-aggregate to form sheet-like structures via intermolecular hydrogen bonds and π–π stacking interactions.


 Please wait while we load your content...
Please wait while we load your content...