A photobasic D–A fluorophore with a high intramolecular charge-transfer as a model strategy for designing organic proton-coupled electron-transfer modulators: an analysis based on steady-state fluorescence, isotopic effect and theoretical study†
Abstract
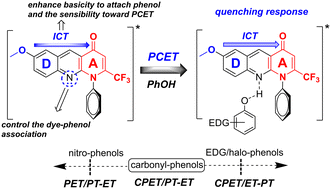
Modulation of the photoinduced proton-coupled electron transfer (PCET) mechanism is a topic of great interest because of its pivotal role in energy-transfer processes, the development of fuel cells and activation of small molecules. Herein we show that the N1-(4-chlorophenyl)-2-(trifluoromethyl)benzo[b][1,8]naphthyridin-4(1H)-one fluorophore is a convenient platform to promote selectively diverse PCET mechanisms upon phenol binding. The key points for the PCET-probe are: (i) the inclusion of an internal basic moiety into the donor (D)–acceptor (A) chain and (ii) the occurrence of intramolecular charge transfer (ICT) upon excitation. Weak fluorescence quenching and detrimental KIEs were observed when a low-CT fluorophoric analogue was used as a PCET probe. The diverse PCET mechanisms upon phenol binding were recognized from a steady-state spectroscopic study, UV-vis spectroscopy, isotopic effect experiments and theoretical calculations. A combined photoinduced electron transfer and PT-ET was identified for nitrophenols, whereas carbonylphenols promoted an apparent combined PT-ET/CPET mechanism with a quenching response dependent on the carbonyl position in the aryl ring. Alkyl-, methoxyphenols, phenol and some aminophenols involved combined CPET and ET-PT mechanisms and halophenols a CPET mechanism, whereas strongly electron-donating hydroxyl- or N-acetamidophenols and 4-aminophenol involved a weak PCET mechanism. The present investigation opens a new perspective for the rational design of selective and effective photobasic organic modulators of PCET mechanisms based on the same principles as typical metallic complexes: (i) a weak base as a hydrogen catcher and (ii) an internal ICT to enhance the basicity of the PCET modulator.
- This article is part of the themed collection: Celebrating Latin American Chemistry


 Please wait while we load your content...
Please wait while we load your content...