Discovery of carboxyl-containing heteroaryldihydropyrimidine derivatives as novel HBV capsid assembly modulators with significantly improved metabolic stability†
Abstract
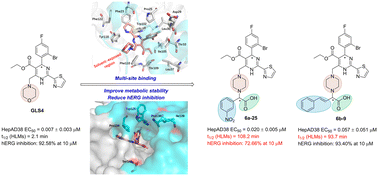
Interfering with the assembly of hepatitis B virus (HBV) capsid is a promising approach for treating chronic hepatitis B (CHB). In order to enhance the metabolic stability and reduce the strong hERG inhibitory effect of HBV capsid assembly modulator (CAM) GLS4, we rationally designed a series of carboxyl-containing heteroaryldihydropyrimidine (HAP) derivatives based on structural biology information combined with medicinal chemistry strategies. The results from biological evaluation demonstrated that compound 6a-25 (EC50 = 0.020 μM) exhibited greater potency than the positive drug lamivudine (EC50 = 0.09 μM), and was comparable to the lead compound GLS4 (EC50 = 0.007 μM). Furthermore, it was observed that 6a-25 reduced levels of core protein (Cp) and capsid in cells. Preliminary assessment of drug-likeness revealed that 6a-25 exhibited superior water solubility (pH 2.0: 374.81 μg mL−1; pH 7.0: 6.85 μg mL−1; pH 7.4: 25.48 μg mL−1), liver microsomal metabolic stability (t1/2 = 108.2 min), and lower hERG toxicity (10 μM inhibition rate was 72.66%) compared to the lead compound GLS4. Overall, compound 6a-25 holds promise for further investigation.

 Please wait while we load your content...
Please wait while we load your content...