Synthesis and pharmacological evaluation of 1,3-diaryl substituted pyrazole based (thio)urea derivatives as potent antimicrobial agents against multi-drug resistant Staphylococcus aureus and Mycobacterium tuberculosis†
Abstract
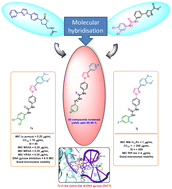
The urgent development of newer alternatives has been deemed a panacea for tackling emerging antimicrobial resistance effectively. Herein, we report the design, synthesis, and biological evaluation of 1,3-diaryl substituted pyrazole-based urea and thiourea derivatives as antimicrobial agents. Preliminary screening results revealed that compound 7a (3,4-dichlorophenyl derivative) exhibited potent activity against S. aureus (MIC = 0.25 μg mL−1) and compound 7j (2,4-difluorophenyl derivative) against Mycobacterium tuberculosis (MIC = 1 μg mL−1). Compounds 7a and 7j were non-toxic to Vero cells with a favorable selectivity index of 40 and 200, respectively, and demonstrated good microsomal stability. Compound 7a exhibited equipotent activity (MIC = 0.25 μg mL−1) against various multidrug-resistant strains of S. aureus, which include various strains of MRSA and VRSA, and elicited bacteriostatic properties. In an enzymatic assay, 7a effectively inhibited DNA gyrase supercoiling activity at a concentration of 8 times MIC. Further, molecular modeling studies suggested that compound 7a binds at the active site of DNA gyrase with good affinity.


 Please wait while we load your content...
Please wait while we load your content...