Antileishmanial evaluation of triazole–butenolide conjugates: design, synthesis, in vitro screening, SAR and in silico ADME predictions†
Abstract
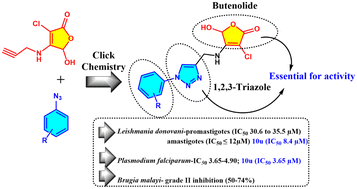
In the quest to discover novel scaffolds with leishmanicidal effects, a series of 23 compounds containing the most promising 1,2,3-triazole and highly potent butenolide in one framework were synthesized. The synthesized conjugates were screened against Leishmania donovani parasite; five of them showed moderate antileishmanial activity against promastigotes (IC50 30.6 to 35.5 μM) and eight of them exhibited significant activity against amastigotes (IC50 ≤12 μM). Compound 10u was found to be the most active (IC50 8.4 ± 0.12 μM) with the highest safety index (20.47). The series was further evaluated against Plasmodium falciparum (3D7 strain) and seven compounds were found to be moderately active. Among them, again 10u emerged as the most active compound (IC50 3.65 μM). In antifilarial assays against adult female Brugia malayi, five compounds showed grade II inhibition (50–74%). Structure–activity relationship (SAR) analysis suggested a substituted phenyl ring, triazole and butenolide as essential structural features for bioactivity. Moreover, the results of in silico ADME parameter and pharmacokinetic studies indicated that the synthesized triazole–butenolide conjugates abide by the required criteria for the development of orally active drugs, and thus this scaffold can be used as a pharmacologically active framework that should be considered for the development of potential antileishmanial hits.


 Please wait while we load your content...
Please wait while we load your content...