Visible and NIR light photoactivatable o-hydroxycinnamate system for efficient drug release with fluorescence monitoring†
Abstract
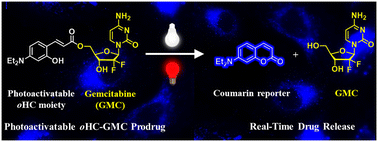
Photoactivatable protecting groups (PPGs) have become powerful materials for controlling the activity of biologically important molecules in the biomedical field. However, designing PPGs that can be efficiently activated by biologically benign visible and NIR light with fluorescence monitoring is still a great challenge. Herein, we report o-hydroxycinnamate-based PPGs that can be activated by both visible (one-photon) and NIR (two-photon) light for controlled drug release with real-time monitoring. Thus, a photoremovable 7-diethylamino o-hydroxycinnamate group is covalently attached to an anticancer drug, gemcitabine, to establish a photoactivatable prodrug system. Upon excitation by visible (400–700 nm) or NIR (800 nm) light, the prodrug efficiently releases drug which is quantified by monitoring the formation of a strongly fluorescent coumarin reporter. The prodrug is taken up by the cancer cells and interestingly accumulates within mitochondria as determined by FACS and fluorescence microscopy imaging. Further, the prodrug demonstrates photo-triggered, dose-dependent, and temporally controlled cell death upon irradiation with both visible and NIR light. This photoactivatable system could be useful and adapted in the future for the development of advanced therapies in biomedicine.


 Please wait while we load your content...
Please wait while we load your content...