Design, synthesis, and biological evaluation of new arjunolic acid derivatives as anticancer agents†
Abstract
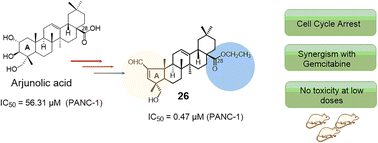
Arjunolic acid (AA) is a pentacyclic triterpenoid with promising anticancer properties. A series of novel AA derivatives containing a pentameric A-ring with an enal moiety, combined with additional modifications at C-28, were designed and prepared. The biological activity on the viability of human cancer and non-tumor cell lines was evaluated in order to identify the most promising derivatives. Additionally, a preliminary study of the structure–activity relationship was carried out. The most active derivative, derivative 26, also showed the best selectivity between malignant cells and non-malignant fibroblasts. For compound 26, the anticancer molecular mechanism of action in PANC-1 cells was further studied and the results showed that this derivative induced a cell-cycle arrest at G0/G1 phase and significantly inhibited the wound closure rate of PANC-1 cancer cells in a concentration-dependent manner. Additionally, compound 26 synergistically increased the cytotoxicity of Gemcitabine, especially at a concentration of 0.24 μM. Moreover, a preliminary pharmacological study indicated that at lower doses this compound did not demonstrate toxicity in vivo. Taken together, these findings suggest that compound 26 may be a valuable compound for the development of new pancreatic anticancer treatment, and further studies are needed to explore its full potential.


 Please wait while we load your content...
Please wait while we load your content...