Design and synthesis of hydrophobic mixed organogels with complementary hydrogen-bond donor–acceptor sites: removal of heavy metal ions Hg2+, Cd2+ and Pb2+ from aqueous solution†
Abstract
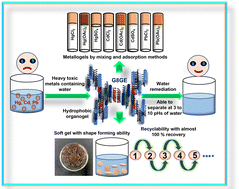
Gels, an intermediate state between a solid and liquid, are attracting attention due to the immense possibilities of their potential applications in various fields. Various non-covalent interactions play essential roles in imparting gel strength and stability. Though incorporating metal ions in gelator systems can generate different kinds of metallogels, capturing heavy metal ions using gel structures has scarcely been investigated by researchers. Previously, one low molecular weight gelator (LMWG) molecule viz. N2,N4,N6-tri(1H-tetrazol-5-yl)-1,3,5-triazine-2,4,6-triamine (G8) was introduced by us for discriminating between various isomers of aminopyridines. However, the gel strength was found to be moderate, and it was unstable in water. Herein, we introduced another molecule viz. 3,3′,3′′-(benzenetricarbonyltris(azanediyl))tris(4-aminobenzoic acid) (GE) along with G8 to impart greater stability and make the mixed-gel water stable. The design of the GE ensures the presence of complementary hydrogen bond donor–acceptor sites with respect to G8. The resultant organogel G8GE has shown the ability to form metallogels with Hg(II), Cd(II), and Pb(II) salts. The hydrophobic nature of the gel G8GE is observed with the retention of the disc-shaped gel structure in an aqueous medium for an extended period. Metallogels were fabricated with two different methods viz. mixing and adsorption methods. Several experiments indicated that in the adsorption method, slow diffusion of metal ions does not disturb the interactions between G8 and GE substantially, proving it to be a more efficient process for gel formation. DFT-based optimization of the structure supports the complementary hydrogen bond formation hypothesis between G8 and GE. The mixed organogel G8GE shows the capacity to efficiently remove chloride, acetate, and sulphate salts of toxic metal ions like Hg(II), Cd(II), and Pb(II) from their aqueous solutions. The xerogel G8GE adsorbs 56.27% of mercury chloride, 99.24% of mercury acetate, 99.90% of mercury sulphate, 51.83% of cadmium chloride, 98.68% of cadmium acetate, 84.47% of cadmium sulphate, 59.70% of lead chloride and 99.90% of lead acetate and the adsorption capacities (qe) are 76.24, 157.79, 147.81, 52.09, 131.25, 284.93, 83 and 221.02 mg g−1, respectively. The adsorbent material, i.e., xerogel G8GE can be reused for five cycles with 97–99% recovery of the xerogel for further water remediation by treating the mercury-contained mixed organogel with excess KI.


 Please wait while we load your content...
Please wait while we load your content...