Iron-catalyzed regioselective C–H alkylation of indoles: an additive-free approach in renewable solvent†
Abstract
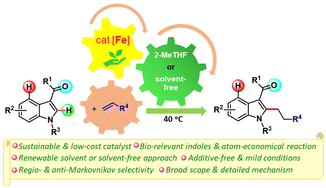
Alkylated indoles are important motifs in various biologically active molecules and drug candidates. Herein, we report a mild and efficient iron-catalyzed protocol for synthesizing alkylated indoles via C–H bond alkylation of indoles with unactivated alkenes, demonstrating a high level of regioselectivity. The reaction occurs under additive-free, solvent-free (or trace green solvent, 2-MeTHF) and less energy-intensive conditions using a sustainable metal catalyst and provides easy access to privileged alkylated indoles with anti-Markovnikov selectivity. Alkylation is compatible with important functionalities, such as fluoro, chloro, trifluoromethyl, alkenyl, ether, thioether, silyl, and siloxane, including heteroaryl, pyridinyl, carbazolyl, and indolyl moieties (45 examples, up to 96% yield). The developed protocol is very simple, straightforward, and fully accords with the principles of green chemistry. A detailed mechanistic investigation manifests the facile indole's C–H activation at the Fe(0) center, reversible 1,2-insertion of the alkene into the Fe–H bond of a metallacycle, and a turnover-limiting reductive elimination.


 Please wait while we load your content...
Please wait while we load your content...