Metal-free, tert-butyl nitrite promoted C(sp2)–S coupling reaction: the synthesis of aryl dithiocarbamates and analysis of antimicrobial activity by ‘in silico’ and ‘in vitro’ methods for drug modification†
Abstract
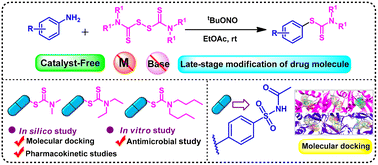
An environmentally friendly, metal-free, and efficient C(sp2)–S coupling reaction protocol between aniline and low-cost tetraalkylthiuram disulfides was developed to synthesize aryl dithiocarbamates. The targets for developing the method included catalyst-free, base-free, mild reaction conditions, room temperature synthesis with high yields, and broad substrate scope. Apart from this, our approach is also helpful for synthesizing potentially bioactive compounds and drug modification. An antimicrobial assay and a docking study on dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) enzymes of the three synthetic derivatives were performed to evaluate the antimicrobial potency of the new compounds and thus inhibit the enzyme's action.
- This article is part of the themed collection: Celebrating the scientific accomplishments of RSC Fellows


 Please wait while we load your content...
Please wait while we load your content...